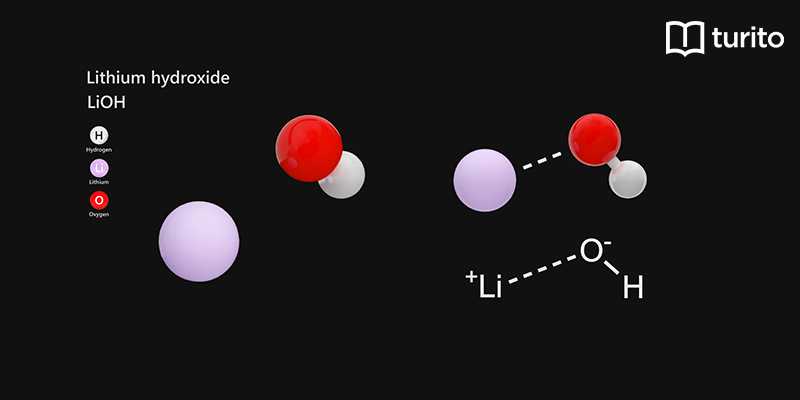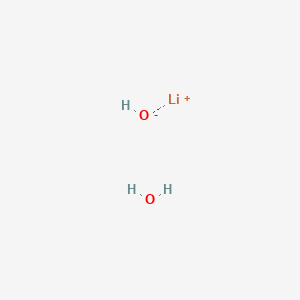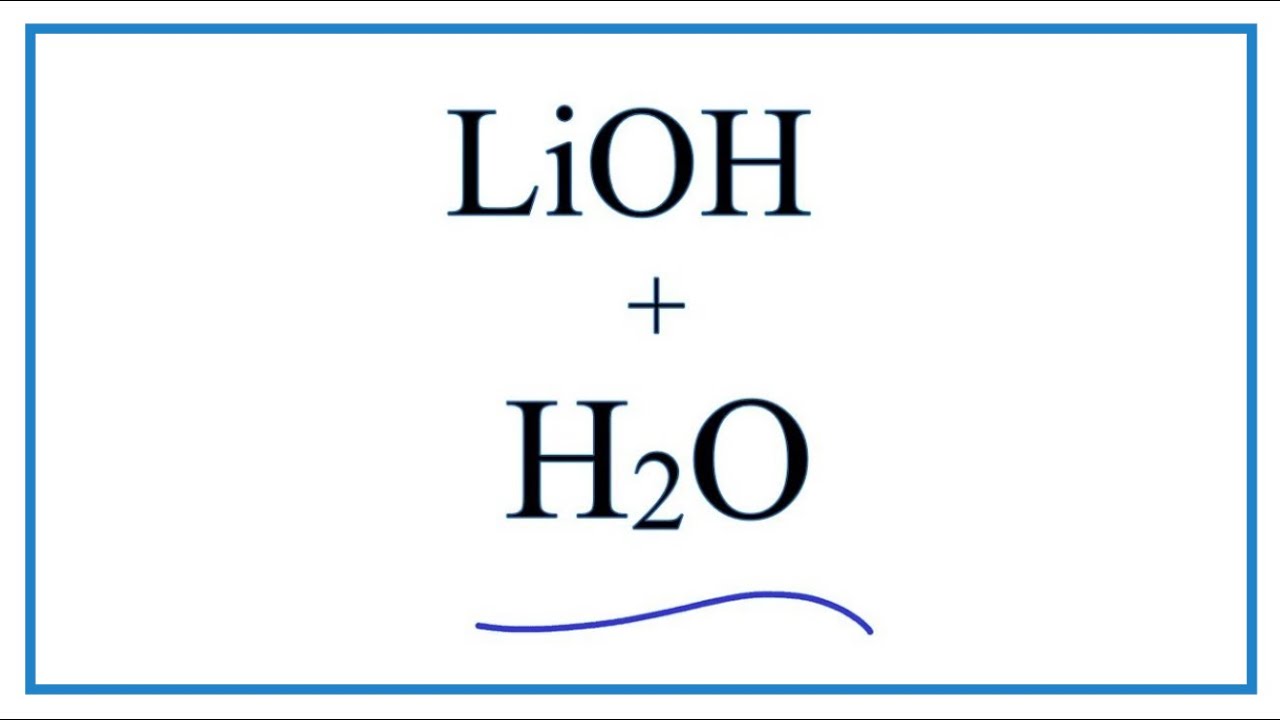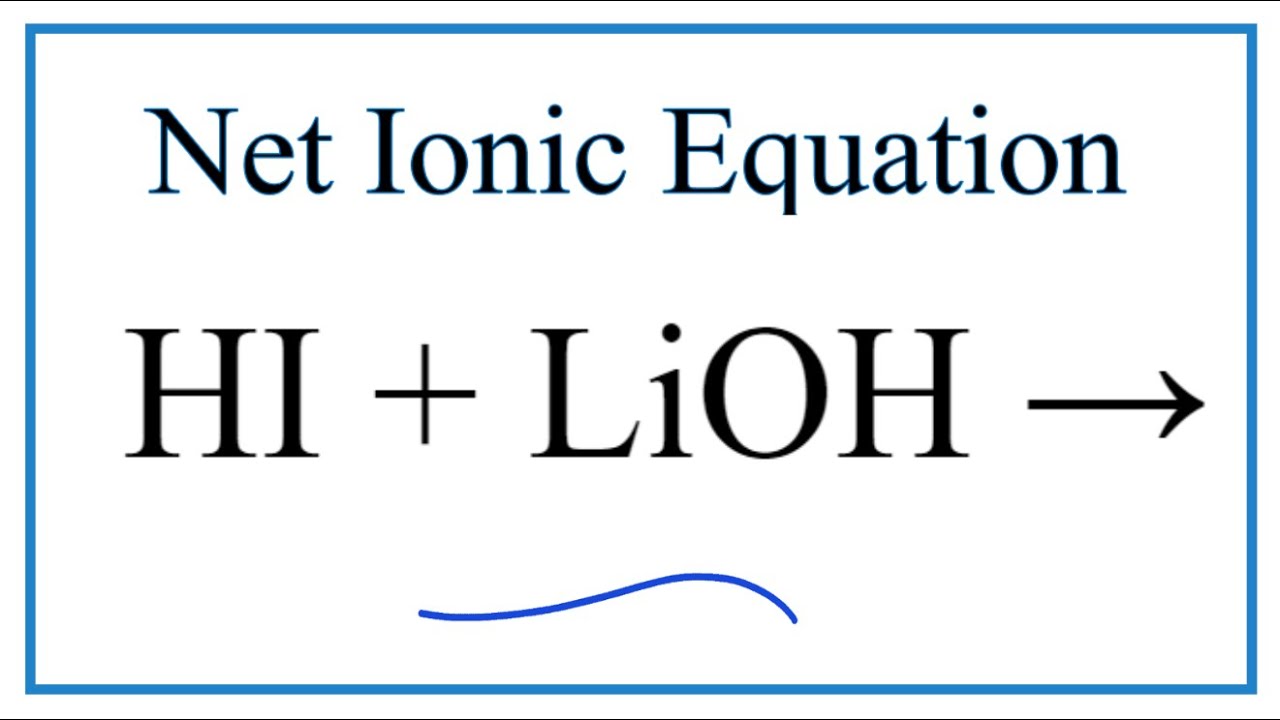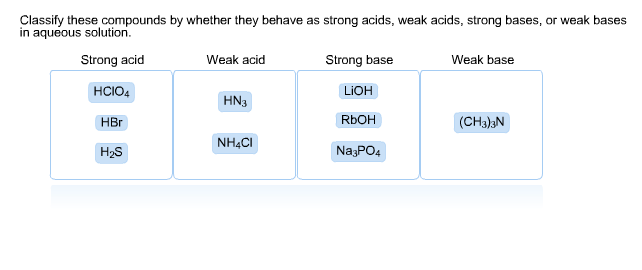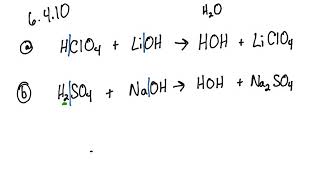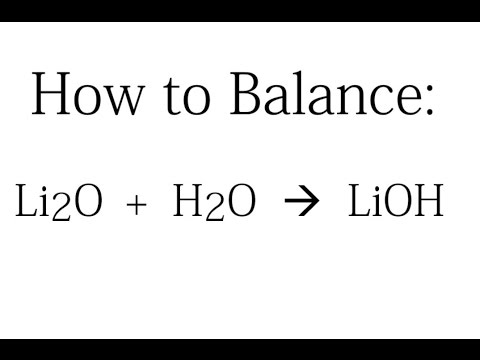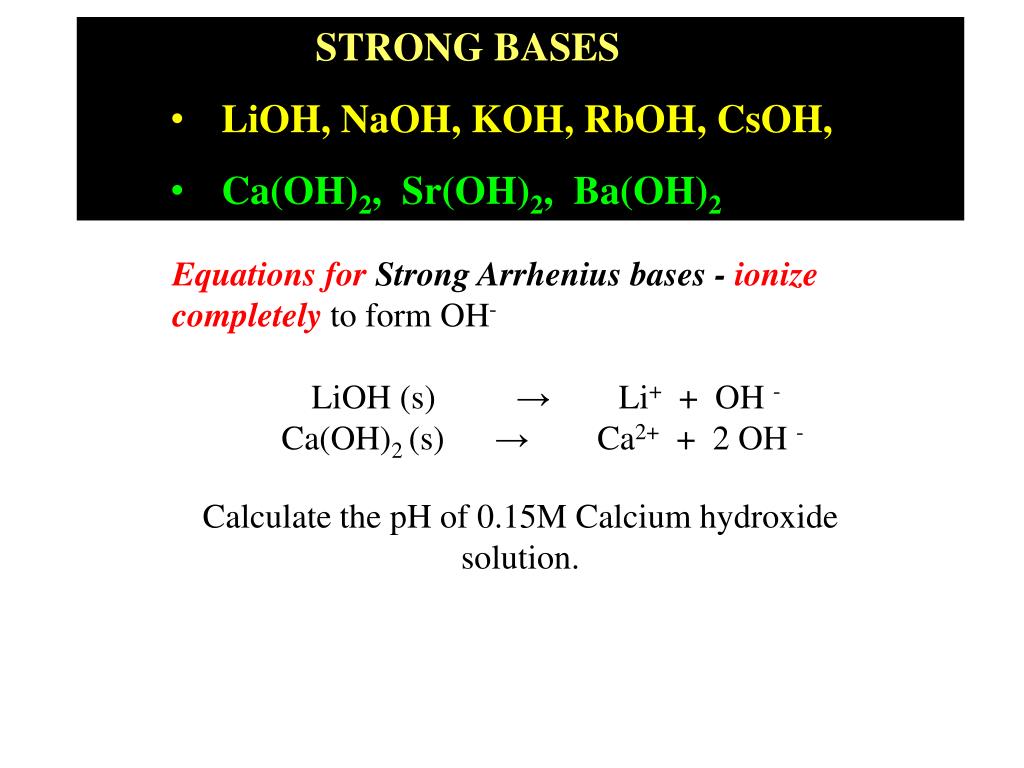
PPT - STRONG BASES LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH) 2 , Sr(OH) 2 , Ba(OH) 2 PowerPoint Presentation - ID:5872807
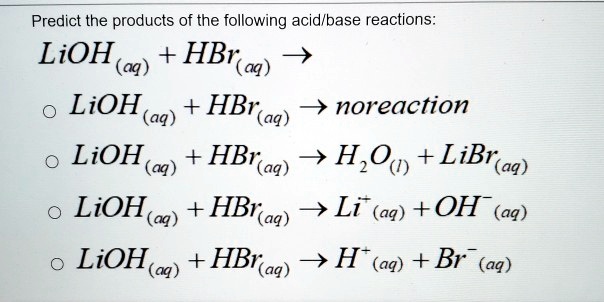
SOLVED: Predict the products of the following acid-base reactions: 1. LiOH (aq) + HBr (aq) â†' no reaction 2. LiOH (aq) + HBr (aq) â†' H2O (l) + LiBr (aq) 3. LiOH (
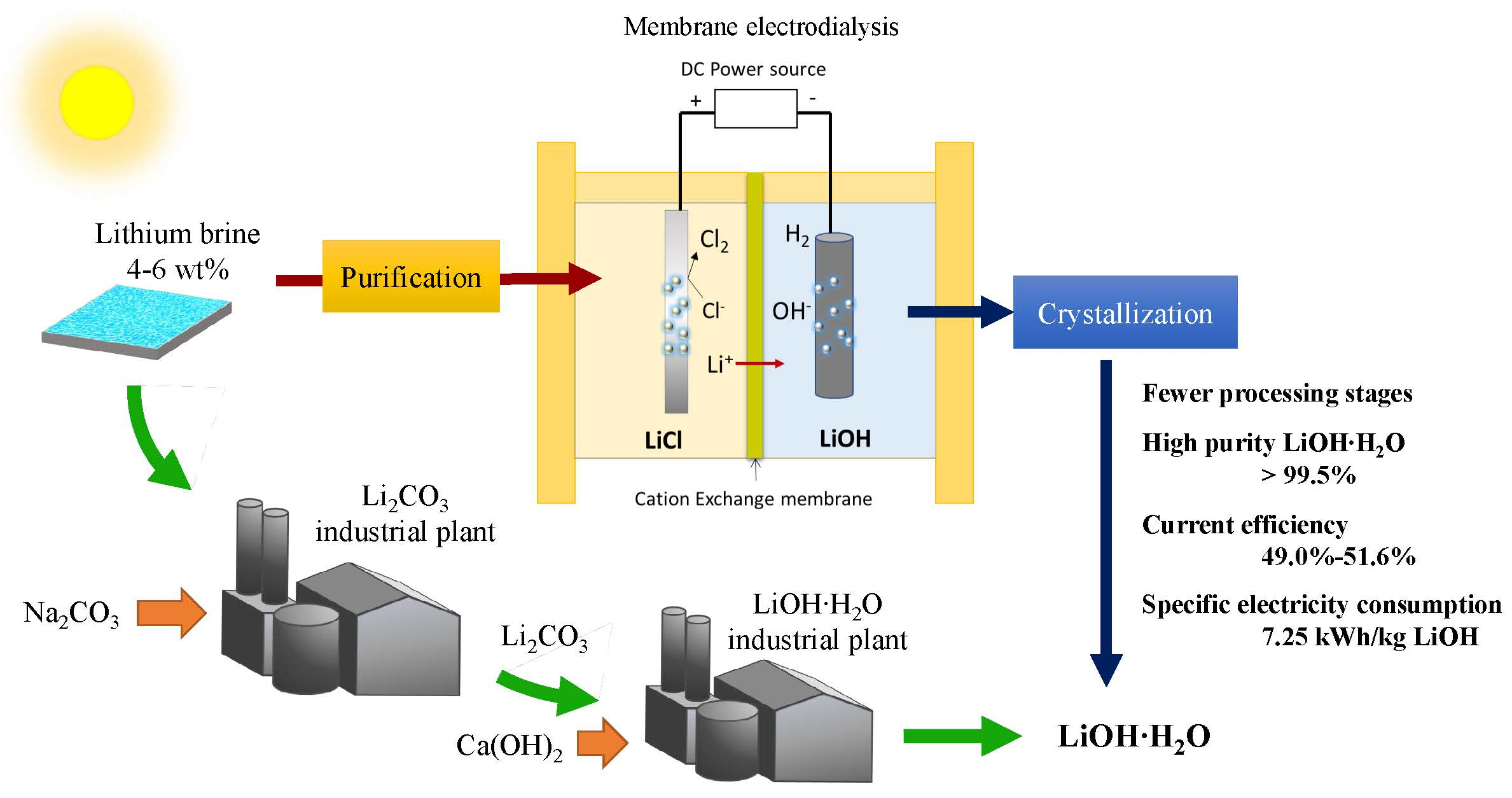
Membranes | Free Full-Text | Analysis of a Process for Producing Battery Grade Lithium Hydroxide by Membrane Electrodialysis

Titration curves for solutions with 1.0 × 10 −4 moles of LiOH·H 2 O and... | Download Scientific Diagram
Equilibrium structures of protonated bases LiOH 2 + , NaOH 2 + , KOH 2... | Download Scientific Diagram
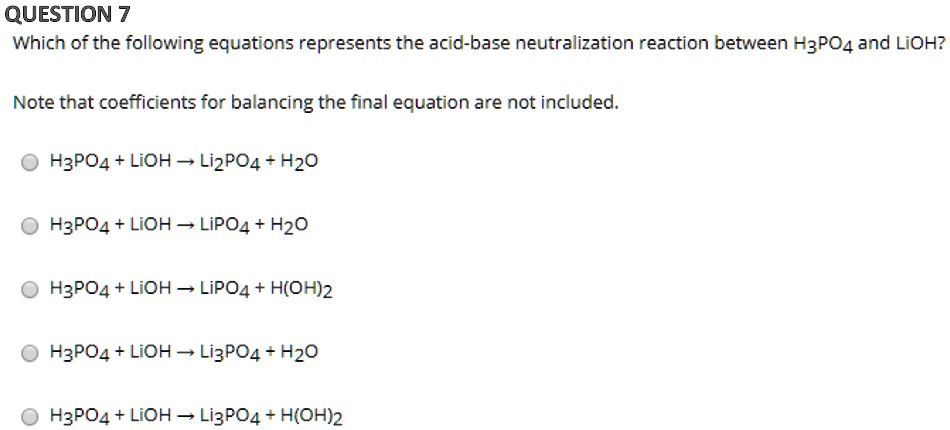
SOLVED: QUESTION 7 Which of the following equations represents the acid-base neutralization reaction between H3PO4 ad LiOH? Note that coefficients for balancing the final equation are not included: H3PO4 + LiOH LizPO4 -