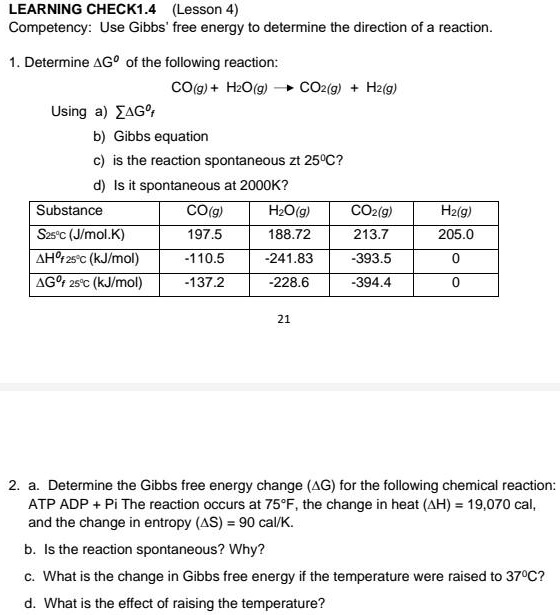
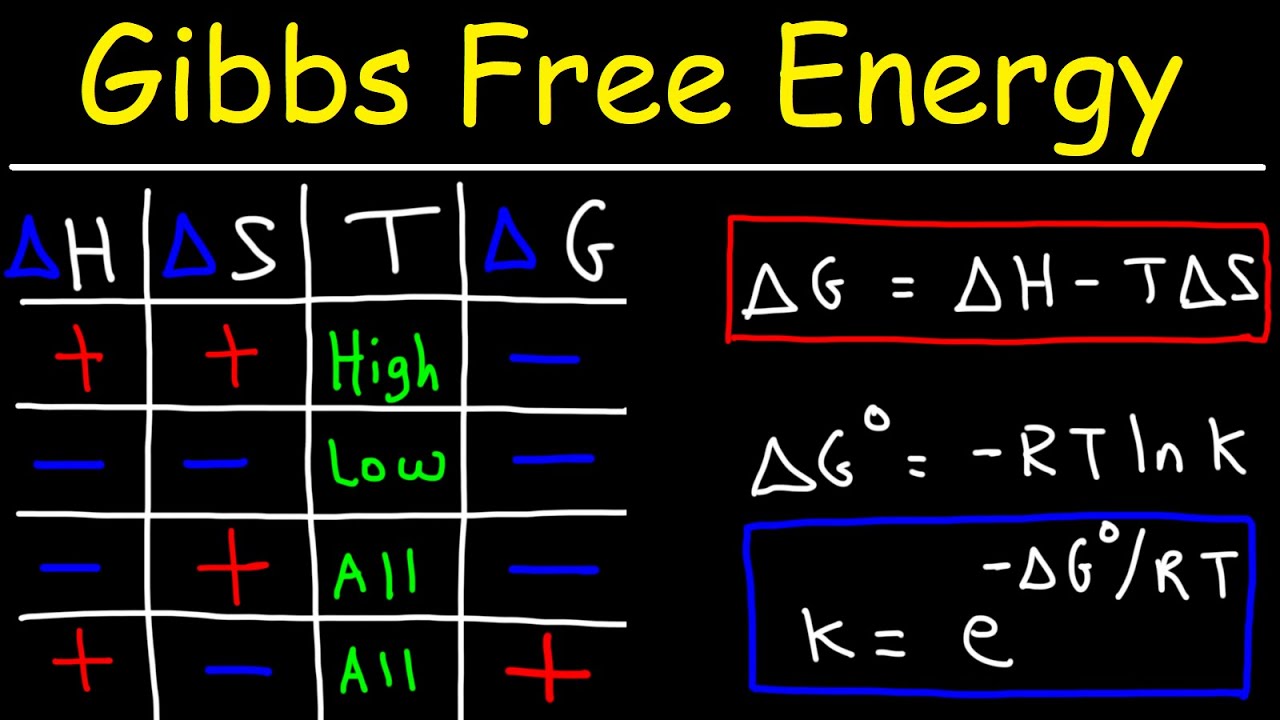
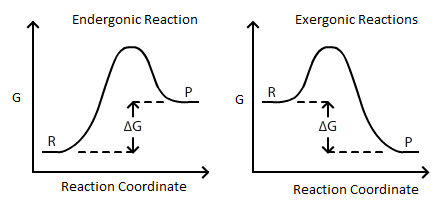
SOLVED: Calculate the standard entropy, Δð '†âˆ˜rxn, of the reaction at 25.0 °C using the table of thermodynamic properties. C2H4(g) + H2O(l) ⟶ C2H5OH(l) Δð '†âˆ˜rxn = Calculate the standard Gibbs free

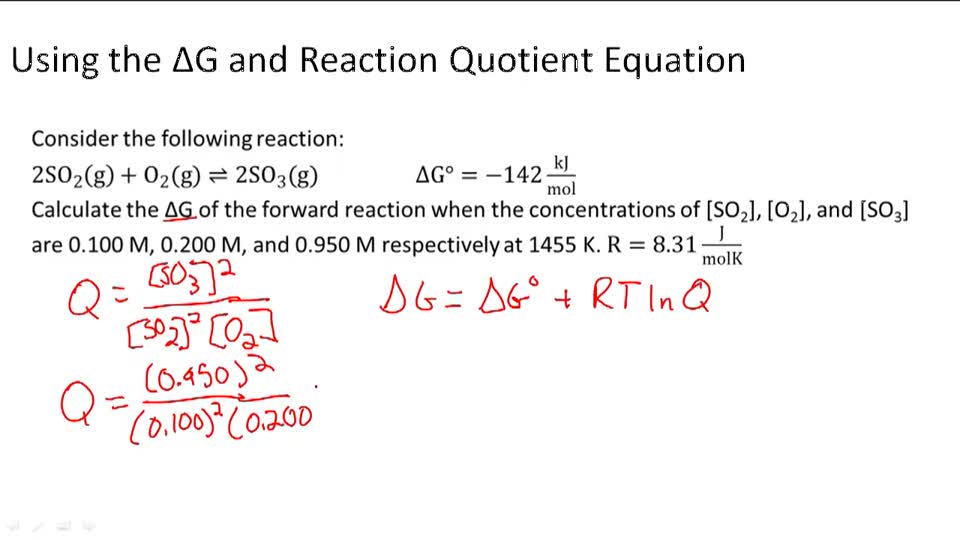
thermodynamics - Reaction quotient and Gibbs free energy at the start of a reaction - Chemistry Stack Exchange

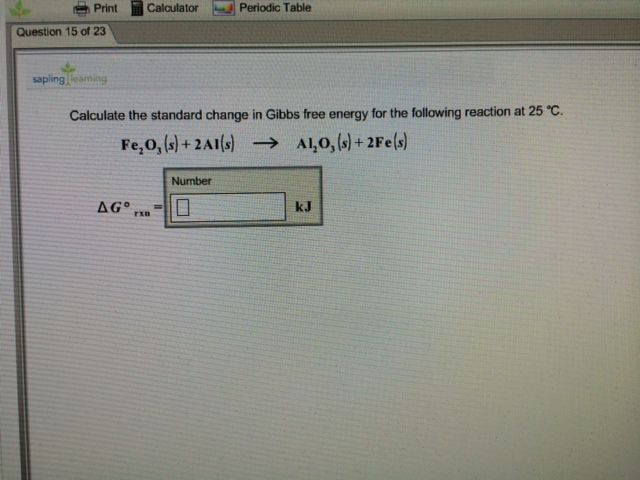
Calculate the standard free energy change for the reaction, 2Ag + 2H^ + ⟶ H2 + 2Ag^ + ; E^0 for Ag^ + + e^ - ⟶ Ag is 0.08 V.