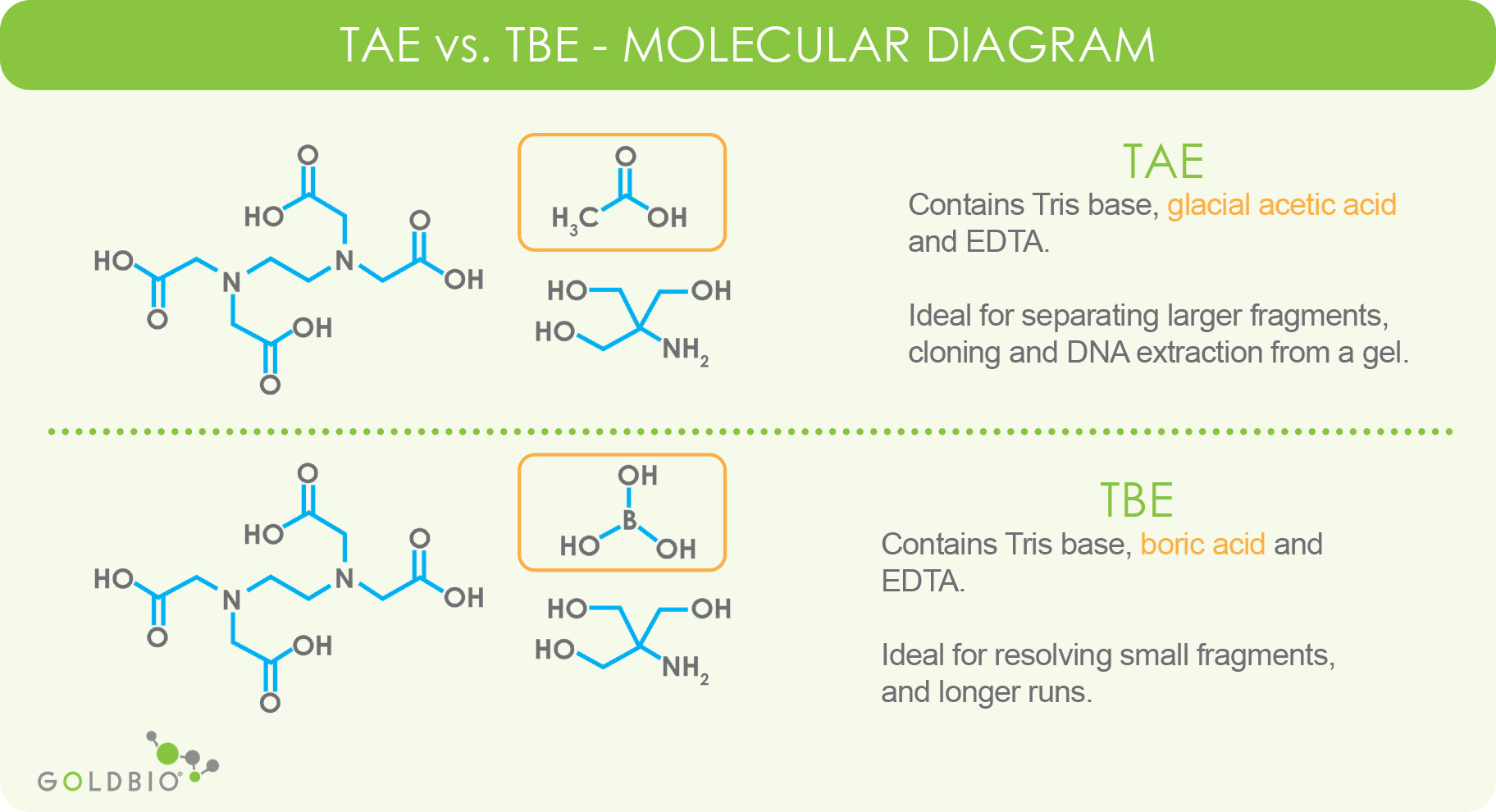
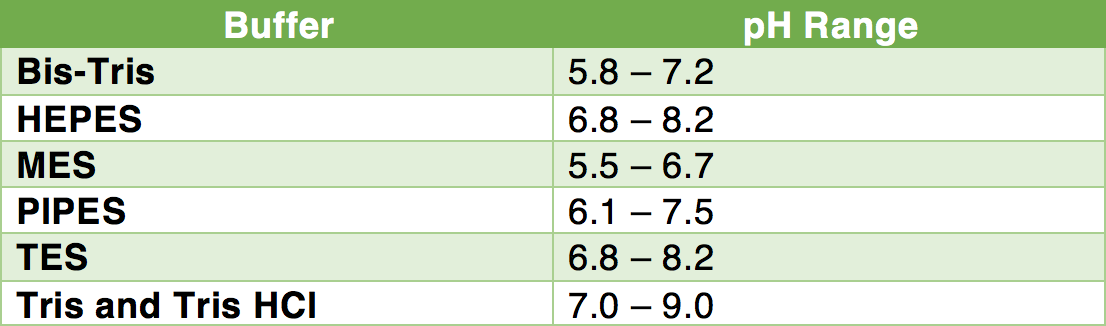
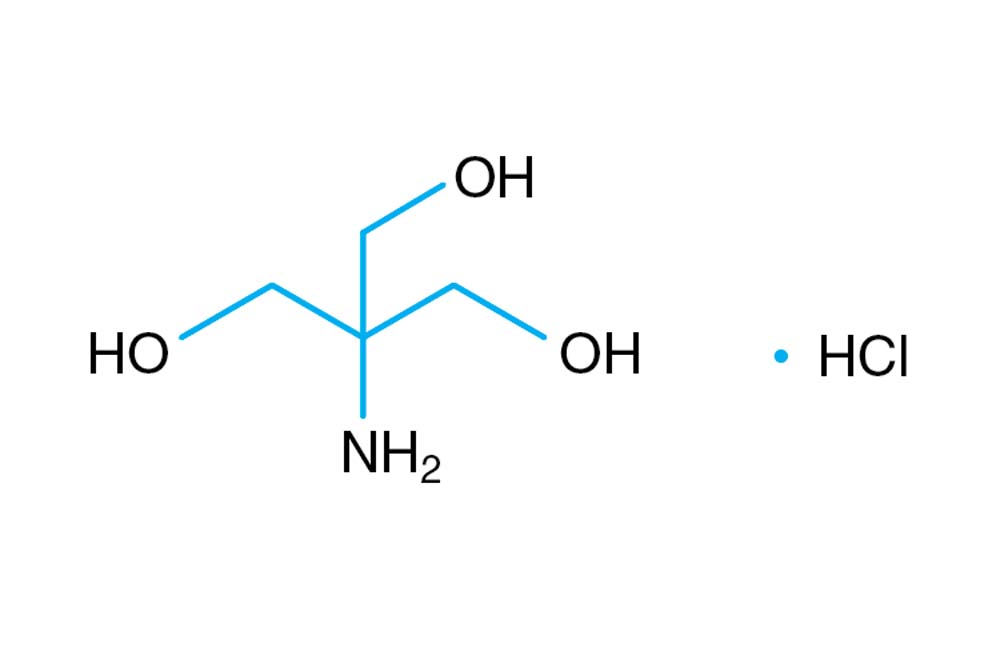
New insights into the effect of Tris-HCl and Tris on corrosion of magnesium alloy in presence of bicarbonate, sulfate, hydrogen phosphate and dihydrogen phosphate ions - ScienceDirect
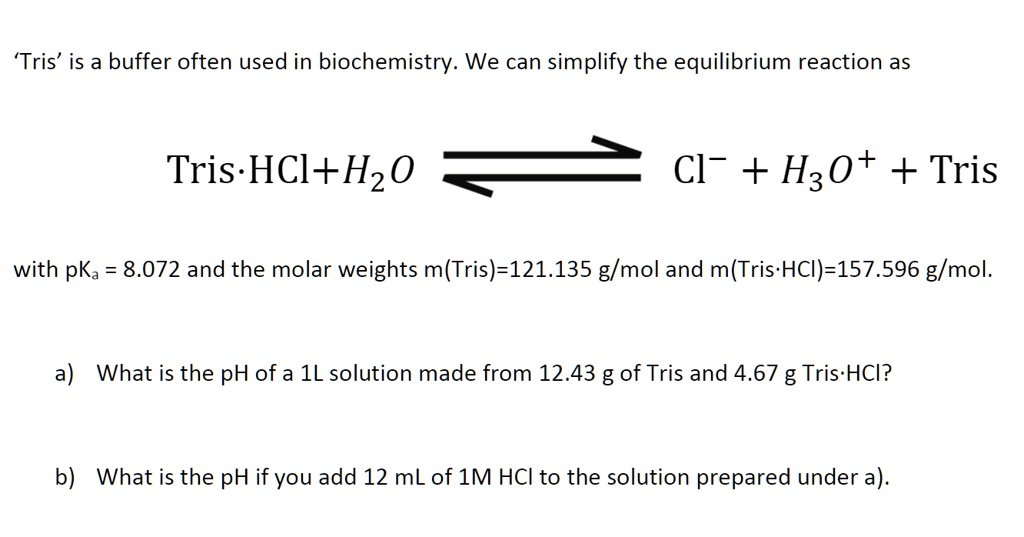
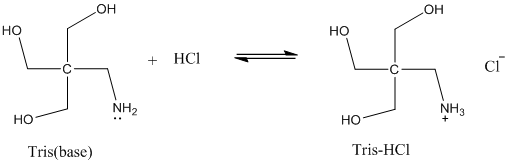
SOLVED: 'Tris' is a buffer often used in biochemistry We can simplify the equilibrium reaction as Tris-HCl+Hz0 Cl- + Hso+ + Tris with pKa = 8.072 and the molar weights m(Tris)-121.135 g/mol
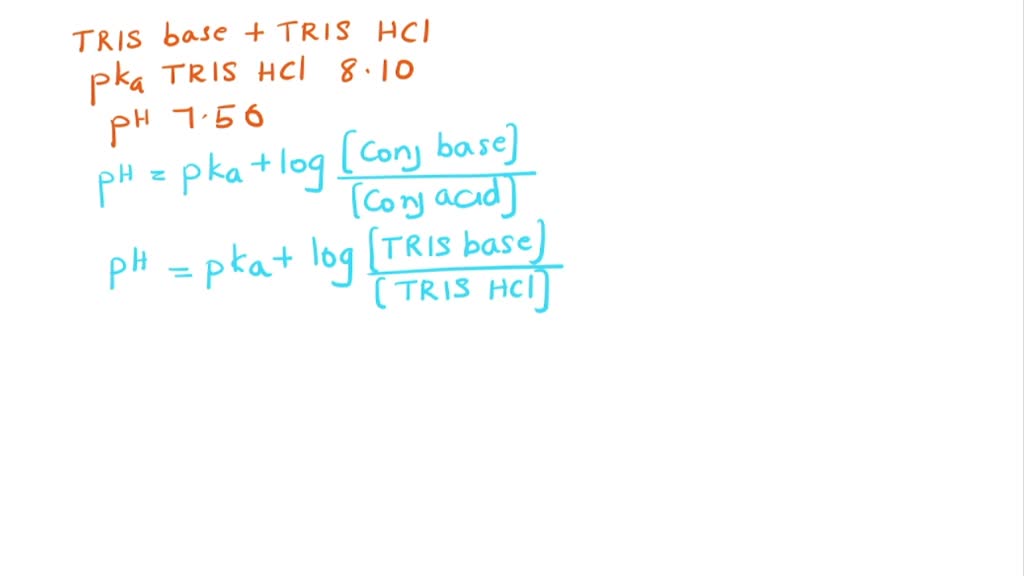
SOLVED: Question 35 3 pts A buffer is made from a mixture of TRIS base and TRIS-HCI The pKa of TRIS-HCl is 8.10. What is the ratio of TRIS base to TRIS -HCI
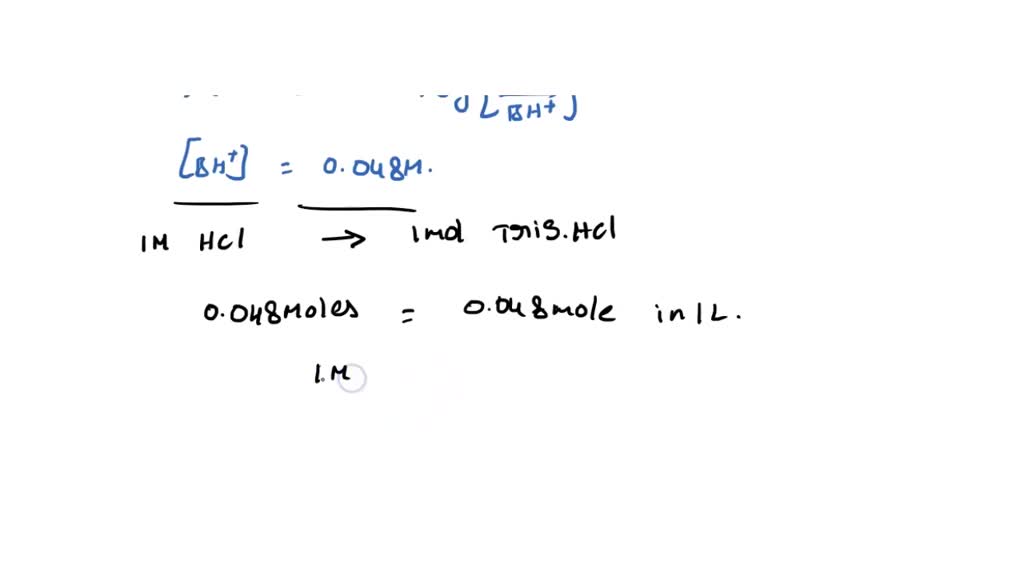



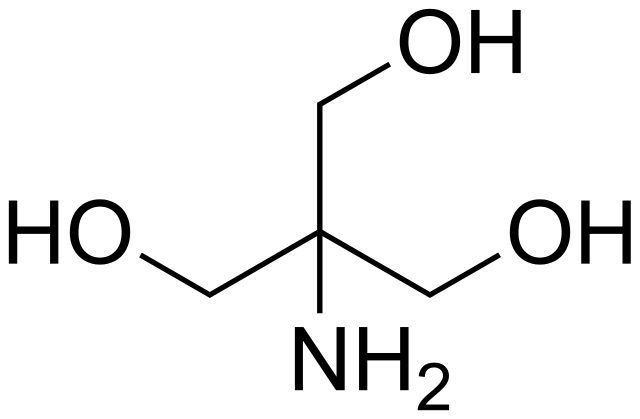
![Tris Base [C4H11NO3] Molecular Weight Calculation - Laboratory Notes Tris Base [C4H11NO3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/11/tris-base-molecular-weight-calculation-300x204.jpg)

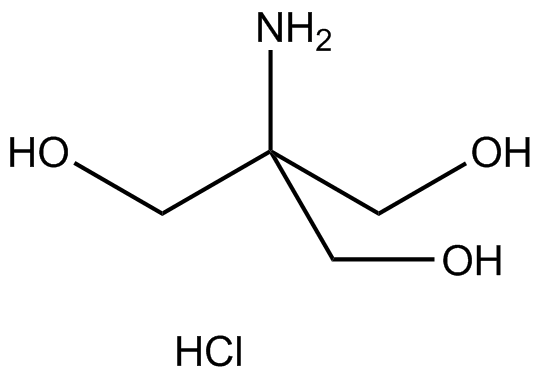
![BT156] 2M Tris-HCl, pH 9.5 | Biosolution BT156] 2M Tris-HCl, pH 9.5 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/07/BT066-Tris-HCl.jpg)